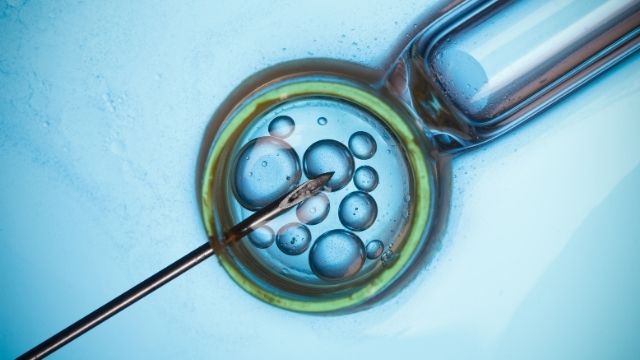
Navigating India’s Assisted Reproductive Technology (ART) Act: Laws, Provisions, and Considerations in Light of Sidhu Moosewala’s Parents’ IVF Pregnancy
Related Articles
Keralam: इतना आसान नहीं है नाम बदलने की प्रक्रिया, जानें कैसे होगा यह बदलाव
Process to change name of state: केरल का नाम बदलने की प्रक्रिया अब औपचारिक रूप से शुरू हो गई है। संविधान के अनुसार, किसी...
12-Year-Old Boy Killed by Minors After Cricket Match Dispute in Delhi
A cricket match in the Tilak Nagar area of West Delhi turned tragic on February 23, when a 12-year-old boy lost his life following...
Coimbatore and Jaipur Rise as Key Hiring Locations for Fresh Graduates in India
In a notable shift, Coimbatore and Jaipur have emerged as primary destinations for freshers, indicating a transformation in the hiring landscape of India. Traditionally,...