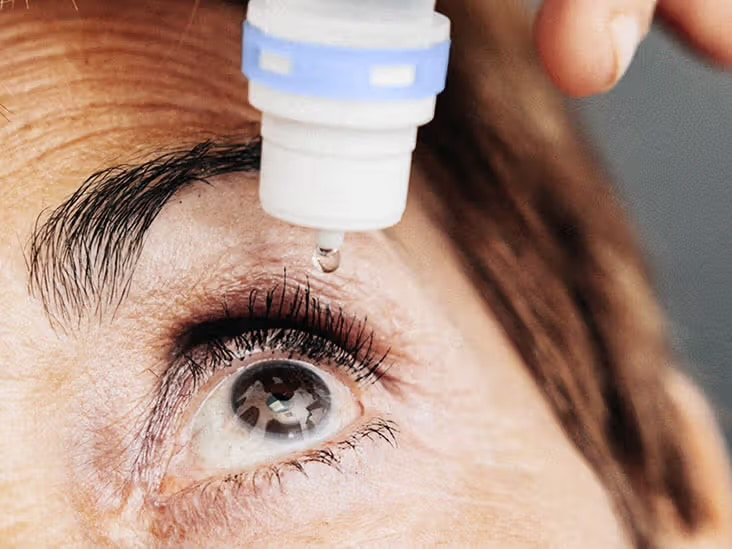
Daily Eye Drop Could End the Need for Reading Glasses by Late 2025
Related Articles
Reliance Industries Announces Rs 10 Lakh Crore Investment Plan for Technology Push
Mukesh Ambani, Chairman of Reliance Industries, has announced a significant investment plan during the India AI Impact Summit 2026. The company is set to...
Kerala High Court Questions CBFC and Producers on Certification of The Kerala Story 2
The Kerala High Court has issued a notice to the Central Board of Film Certification (CBFC) and the producers of The Kerala Story 2...
Two Indian Workers Injured in Suspected Racial Attack in Israel; Accused Held
Two Indian expatriates reportedly suffered injuries in a targeted assault in Ashkelon, Israel, which authorities have described as racially motivated. Reports indicate that the...