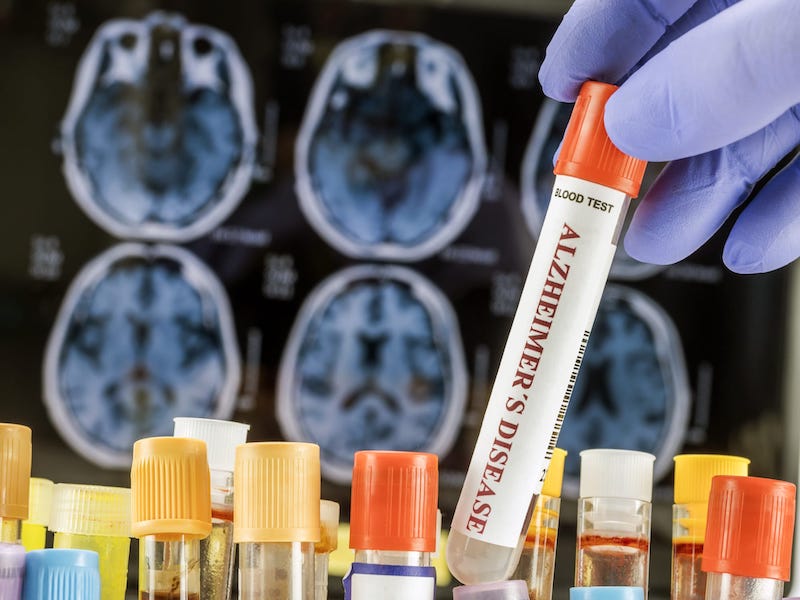
Forgetfulness? New AI Blood Test Catches Alzheimer’s Early in India
Related Articles
Kuwait Imposes Restrictions on Fishing Boats and Outdoor Gatherings Amid Regional Tensions
Kuwait's Interior Ministry has announced the imposition of temporary restrictions on fishing boats and outdoor activities in response to heightened regional tensions.
This decision is...
खामेनेई पर अंकुश: सीएम उमर अब्दुल्ला को सांसद आगा रूहुल्लाह मेहदी का तीखा हमला
नेशनल कॉन्फ्रेंस के सांसद आगा रूहुल्लाह मेहदी ने जम्मू-कश्मीर के मुख्यमंत्री उमर अब्दुल्ला पर हमलावर रुख अपनाया है। उनके मुताबिक, अब्दुल्ला ने ईरान के...
Flu Shot Set for Big Change as Fast-Spreading H3N2 Strain Raises Alarm
In light of a notable surge in flu cases globally, India is set to revise its seasonal influenza vaccine to more effectively address a...