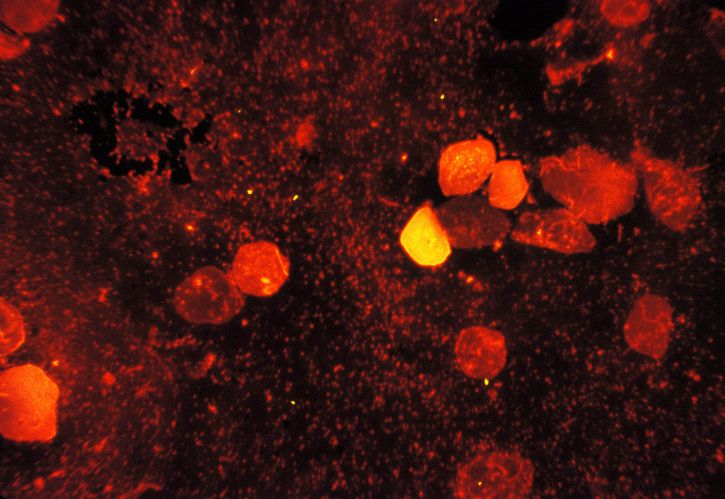
Johnson & Johnson Announces 10-year Initiative to Help End Tuberculosis
Related Articles
Shakira to Headline Feeding India Concert 2026 in Mumbai and Delhi
Shakira, a four-time Grammy Award winner, is set to perform in India as the headliner of the 2026 Feeding India Concert. This concert will...
Balancing Work and Home: A Mother’s Journey to Mental Well-Being
For many working mothers, the end of the office day does not signify a break but marks the beginning of an often-overlooked responsibility at...
As Ramadan 2026 Approaches, a Balanced Suhoor is Vital for Maintaining Energy During Fasting, Emphasizing Hydration and Nutrition
Fasting during the holy month of Ramadan is recognized as both a spiritual and physical experience. As Ramadan approaches in 2026, the significance of...